- Doses of an investigational Marburg vaccine have been sent to Rwanda to combat outbreak
- Texas Biomed partnered with Sabin Vaccine Institute to perform nonclinical testing of the Marburg vaccine
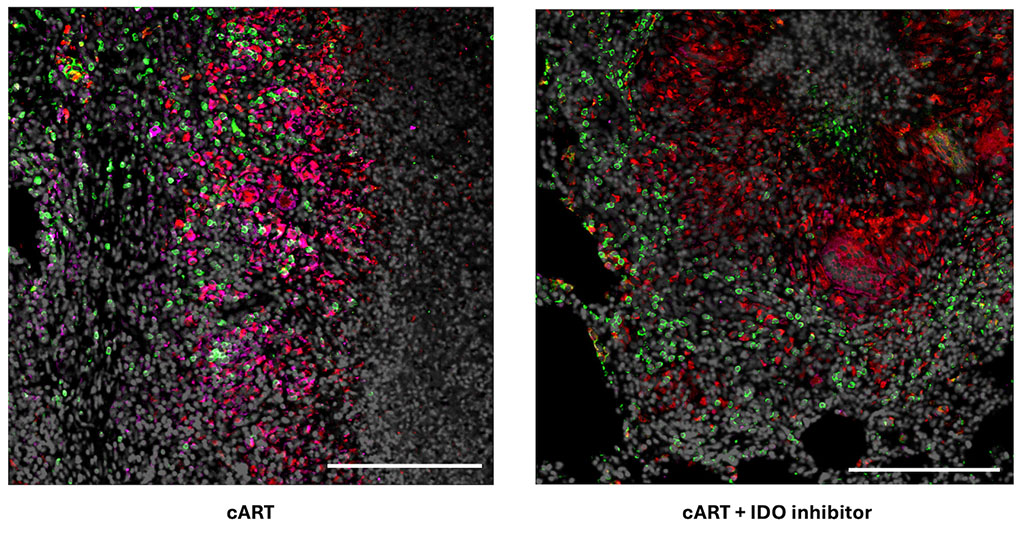
SAN ANTONIO (Oct. 16, 2024) – Texas Biomedical Research Institute (Texas Biomed) conducted early tests of the investigational Marburg vaccine now being deployed to Rwanda to help control the outbreak that began in late September.
The vaccine candidate is being developed by Sabin Vaccine Institute, which delivered 1,700 doses in two separate shipments to Rwanda – the first within nine days of the outbreak being declared.
As of October 15, the Marburg virus outbreak in Rwanda has infected 62 people and caused 15 deaths, according to the Rwandan Ministry of Health. Marburg is part of the same family of viruses as Ebola virus, and similarly causes hemorrhagic fever and has a fatality rate of up to 88%.
“We are grateful that our partners at Sabin have been working to develop a candidate Marburg vaccine so that we can protect people from this very deadly virus,” says Ricardo Carrion, Jr., Ph.D., the Director of Maximum Containment Contract Research at Texas Biomed. “We are proud to have played a role in the studies needed to advance the vaccine to human trials.”
Early tests demonstrating safety and efficacy were completed at Texas Biomed and elsewhere in animal models, which are essential before any vaccine or therapy progresses to clinical trials in humans.
There is currently no approved vaccine or treatment for Marburg virus. Sabin’s vaccine candidate is currently in Phase 2 clinical trials in Kenya and Uganda. At the request of Rwandan officials, Sabin mobilized quickly to provide vaccine doses and partner with the Rwanda Biomedical Centre to launch a rapid response Phase 2 open-label study there. The aim is to vaccinate adults at the highest risk, beginning with healthcare providers, according to a Sabin press release. Rwanda health officials report more than 770 vaccine doses have been administered as of October 15.
“Infectious disease outbreaks are unfortunately occurring more frequently. Texas Biomed is committed to developing countermeasures like vaccines and antibody therapies proactively, to ensure they are ready to be deployed as quickly as possible when an outbreak is declared,” says Cory Hallam, Ph.D., Executive Vice President, Applied Science and Innovation at Texas Biomed. “We applaud our partners for the global cooperation that has seen them launch important clinical trials so quickly after the outbreak was confirmed.”
Research and development of the Sabin vaccine candidate have been largely funded by the U.S. Biomedical Advanced Research and Development Authority (BARDA). Sabin also acknowledges NIAID’s Vaccine Research Center and its manufacturing partner, ReiThera, for their continued support.
More Information
Marburg vaccine tested at Texas Biomed moves to Phase 2 clinical trials
Sabin Vaccine Institute Delivers Marburg Vaccines to Combat Outbreak in Rwanda
Funding information:
This project has been funded in whole or in part with Federal funds from the Department of Health and Human Services, Biomedical Advanced Research and Development Authority, Office of the Assistant Secretary for Preparedness and Response, Office of the Secretary under Contract No. 75A5011900055 and 75A50123C00010.
###
About Texas Biomed
Texas Biomed is a nonprofit research institute dedicated to protecting the global community from infectious diseases. Through basic research, preclinical testing and innovative partnerships, we accelerate diagnostics, therapies and vaccines for the world’s deadliest pathogens. Our San Antonio campus hosts high containment laboratories and the Southwest National Primate Research Center. Our scientists collaborate with industry and researchers globally, and have helped deliver the first COVID-19 vaccine, the first Ebola treatment and first Hepatitis C therapy.