In findings that have implications for potential new HIV therapies, researchers from Texas Biomedical Research Institute (Texas Biomed) used genetic sequencing techniques on the nonhuman primate version of the virus to identify that lymph nodes in the abdomen are the leading source of rebound infection after the first week of stopping antiretroviral treatment.
The study regarding simian immunodeficiency virus (SIV) was reported in the journal Science Translational Medicine. SIV is very closely related to HIV and is commonly used as a proxy to study HIV in animal models.
“Lymphoid tissues are known to be large reservoirs of latent HIV,” says Texas Biomed Professor Binhua “Julie” Ling, MD, PhD, and senior paper author. “However, there has been no definitive proof that they are the source of initial viral rebound – it has been a hypothesis. Now, we have evidence that SIV, and therefore potentially HIV, is hiding in specific types of lymph nodes and spleen tissues and is some of the first to reemerge in blood when treatment is stopped.”
Antiretroviral therapy (ART) does an excellent job at suppressing HIV to undetectable levels in the blood. However, small amounts of latent virus hide throughout the body, including in the brain, lung, gut, spleen, lymph nodes and other organs. When treatment is stopped, it opens the door for the virus to rebound.
“If we can identify the starting point of the virus rebound, we can work on developing treatments that target those tissues and stop the virus from spreading in the first place,” Dr. Ling says.
Dr. Ling and her team used several advanced genetic tools and sequencing techniques to track the virus. They teamed up with Brandon Keele, PhD, at the AIDS and Cancer Virus Program at Fredrick National Laboratory, who generated barcoded viruses. More than 9,000 individual viruses in the stock have unique genetic barcodes, “like when you go to Walmart and each item has its unique barcode to scan,” Dr. Ling explains.
Those barcoded viruses were given to seven nonhuman primates. After infection was established, the primates began receiving antiretroviral therapy. After four to six months on ART, the animals had either very small amounts or no detectable virus circulating in blood, much like people living with HIV who are on ART. When treatment was stopped after more than a year of ART, researchers were able to assess the very earliest stages of viral rebound.
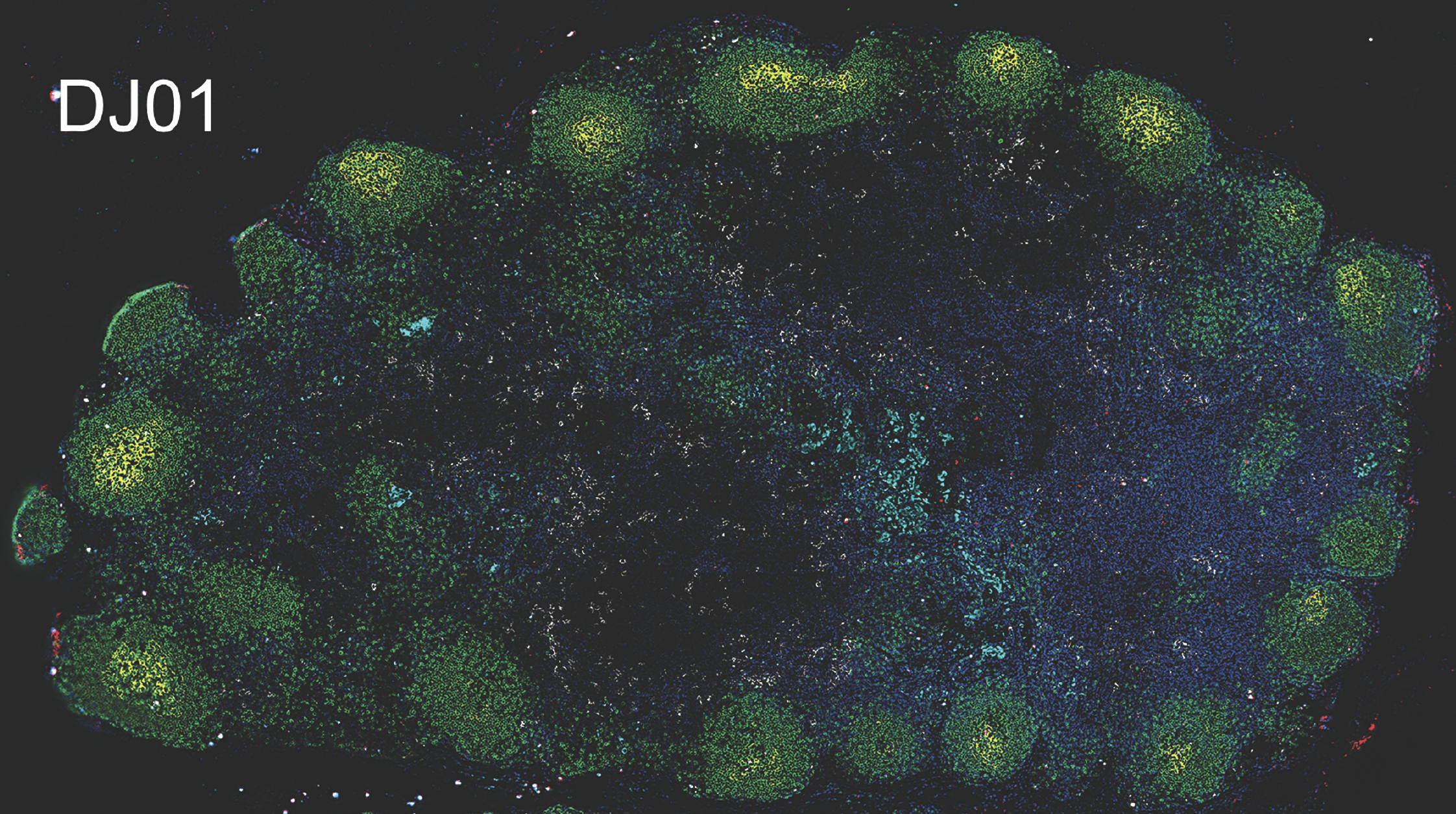
Thanks to the barcoded viruses, they could identify in which tissues the virus had replicated the fastest and spread the furthest just seven days after treatment was stopped. They matched the barcodes most prevalent in blood plasma to the barcodes detected in specific tissues. Notably, the standard test did not detect any virus in blood at the seven-day mark – the amounts present were too low to be detected – but more sensitive deep sequencing did.
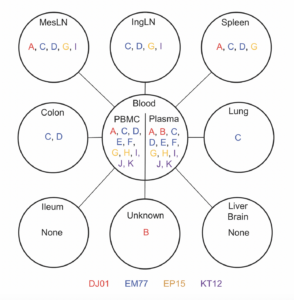
The researchers found three leading contributors: mesenteric lymph nodes, which are found in the tissue connecting the intestines to the abdominal wall; the spleen, which is a part of the lymphatic system that filters blood; and inguinal lymph nodes, which are located in the groin.
Through additional analyses, the researchers found CD4 T cells, a type of immune cell, in the mesenteric lymph nodes and spleen had higher amounts of intact virus and replication activity, which corresponded with higher rates of barcoded viruses from those regions in blood plasma. This was further confirmed using a novel technology by Qingsheng Li, PhD, at University of Nebraska-Lincoln.
Interestingly, some animals showed no evidence of viral rebound, indicating they had better control of the virus in the first week after treatment was stopped than others. Through single cell sequencing and transcriptomic analyses, the team identified a few genes of interest that contribute to dysregulation of normal cell function and could play a role in the differences between animals who experienced very rapid rebound and the animals who continued to suppress viral activity. The researchers are interested in learning more about these genes and how they might affect human immune responses.
The researchers acknowledge that the study size of seven animals is small, and that tissue sample size was also limited. Nonetheless, the results point to key organs to explore specific, targeted therapies.
“There are more than 800 lymph nodes throughout the body,” says Dr. Ling. “Knowing which types of lymph nodes to target can lead to more tailored therapies or treatments and hopefully prevent HIV from spreading and prolong HIV remission.”
Paper:
Antonio Solis-Leal et al., Lymphoid tissues contribute to plasma viral clonotypes early after antiretroviral therapy interruption in SIV-infected rhesus macaques. Sci. Transl. Med.15,eadi9867(2023). DOI:10.1126/scitranslmed.adi9867